condensation
Condensation can be:
in general: densification; squeezing
in physics:
the process by which a substance changes from the gas phase to the liquid phase, see liquefaction
in a broader sense: the transition of a substance to a "dense" state, i.e. from a gas to a solid or liquid state, or e.g. concentration of the solution by evaporation of the solvent
in astronomy: condensation of interstellar matter into a star due to its own gravity and the repulsive pressure of radiation from surrounding stars, see condensation (astronomy)
in chemistry: a reaction in which a polymer is formed from several monomers by splitting off a simple substance (e.g. water), see condensation reaction
in computer science: reorganization of a library consisting of the physical elimination of invalid programs or data files, see condensation (computer science)
in linguistics: condensing sentences by using a semiclause instead of a subordinate clause, see condensation (linguistics)
Gaseous to liquid transformation • is the opposite of evaporation • occurs at the dew point temperature • can occur as the gas cools if the gas particles are close enough to each other and condensation nuclei are present. • during condensation, a gaseous substance transfers heat to its surroundings.
The principle
Condensation is a method of removing solvent vapors from the waste gas stream by lowering the temperature below the dew point. Depending on the temperature range, there are different condensation methods:
condensation with a cooling medium up to a condensation temperature of 25 º C,
condensation with refrigerant up to a condensation temperature of 2 º C,
condensation with brine down to a condensation temperature of -10 ºC,
condensation with ammonia brine up to a condensation temperature of -40 ºC (one degree), or –60 ºC (two degrees),
cryogenic condensation down to a condensing temperature of -120 ºC (in practice often from -40 to -80 ºC in a condensing device),
condensation with a closed inert gas cycle.
Condensation is carried out using direct or indirect cooling. Direct cooling involves direct contact between gas and cooling liquid, while indirect cooling is provided through a heat exchanger. Indirect condensation is preferred because direct condensation requires an additional separation stage. Condensing systems vary from simple stand-alone condensers to complex, multi-condenser systems designed to maximize energy and vapor recovery.
Condensation with a closed cycle of inert gas is intended for the purification of gases with a high vapor concentration. A constant volume of inert gas, usually nitrogen, is continuously recycled around the heating and condensing unit. A portion of the nitrogen-steam mixture flows continuously into the treatment module where a set of heat exchangers cools and condenses the steam.
The design and operation of condensers is highly dependent on the cooling medium used in the process. Two types of heat exchangers are used in liquid-cooled condensing plants:
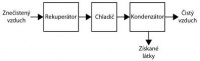
Conventional boiler heat exchanger that is cooled either by water or air. Condensation efficiency can be increased by two-stage operation, using water in the first stage and a cooled liquid (water, brine, etc.) as the cooling medium in the second stage. A two-stage system consists of these parts
recuperator, in which a flow of cooled purified gas is used as a cooling agent,
a cooler for further cooling of the gas using a stream of frozen water or cooled purified air,
main condenser,
valves and tubes.